This page contains the chemical processes involved with making Biodiesel. If you want to know how to make Biodiesel at home then please click this link
Making Biodiesel – The Process
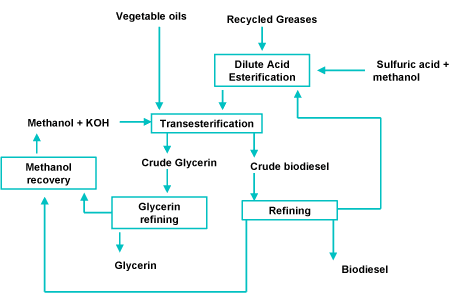
The process of making biodiesel is known as transesterification. This process is achieved by adding methanol to vegetable oil. The process requires a catalyst to increase the rate of the chemical reaction between the methanol and vegetable oil. The catalyst used in the creation of biodiesel is an alkaline. This can be either either Potassium Hydroxide or Sodium Hydroxide.
When the transesterification process is complete the catalyst can be recovered completely unaffected by the chemical reaction that it helped accelerate. This is along with the glycerol separated from the vegetable oil.
How Biodiesel is made
If waste vegetable oil is used you also have to consider that it have been been reheated several times during the course of its use. Reheating causes some of the fatty acids to bond to the glycerol then break away and float freely in the vegetable oil – hence the name Free Fatty Acid (FFA) . There are two ways of dealing with free fatty acids:
1. Esterify the FFAs creating methyl esters then proceeding with the transesterification.
2. Increase the amount of catalyst in the transesterifaction process so that the additional catalyst neutralises the Fatty Free Acids – creating soap as an additional by-product.
Option 1 is used in the commercial production of biodiesel. For smaller scale production option 2 is favoured as it reduces the complexity of the whole process. If using option 2, we would have to perform a titration on a sample of the waste vegetable oil in order to calculate the amount of additional catalyst required to neutralise the Fatty Free Acids.
Ths additional catalyst would then react with the Fatty Free Acids creating soap in the process.
Transesterification is a reversible reaction. This means that the process is working both ways simultaneously until a balance between the vegetable oil and biodiesel is reached. Consequently we need to ensure that the process continues the creation of biodiesel rather than stall once it reaches this point of equilibrium.
In commercial production we would tap off the output as it is created thus ensuring that there is a greater quantity of input vegetable oil to keep the reaction producing the biodiesel. For smaller scale production it is more practical to use an increased volume of methanol to ensure that the reaction continues in the direction of producing biodiesel.
[ad]
Like the catalyst, this excess methanol will be left over after completion of the reaction.